- The Vitruvian Plant: Powering the Future #10-2284
-
Background
History of Nuclear Energy Nuclear Agencies and Regulations Nuclear Power in the Public Eye Economics of Nuclear Energy Radioactivity Basics Nuclear Reactor Basics Current Reactor Designs Processing Radioactive Materials Remote Handling Uses of Radiation Archimedes Filter Technology New Generation Reactors
- Components
- Project
Uses of Radiation
Useful Radioisotopes Found in Spent Nuclear Fuel
| Isotope | Radiation | Half Life | Uses |
| Cs-137 | alpha, beta | 30 years | studying soil erosion, calibration of detecting devices |
| Cs-135 | alpha, beta | 2.0 million years | potential use in ion propulsion engines |
| Sr-90 | beta | 28 years | atomic batteries, SNAP power source, medical radionuclides |
| Am-241 | alpha | 458 years | smoke detectors |
| Am-243 | alpha | 7,950 years | No know domestic use |
| Tc-99 | beta, gamma | 210,000 years | bone imaging, medical tracers |
| Pu-239 | alpha | 24,390 years | fuel for fast breeder reactors |
| Pu-240 | alpha | 6,580 years | No know domestic use |
| Np-237 | alpha | 2.2 million years | possible reactor fuel or military use |
The above list is based on the following chart of predominate radionucluides present in uranium decay.
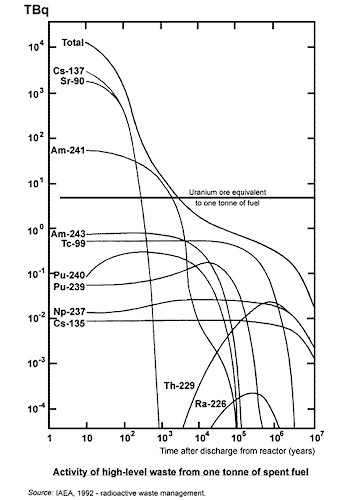
Image courtesy of world-nuclear.org
Atomic Batteries and Betavoltaics
The idea
of atomic batteries has been around for over 100 years. They seek to use charged
particles released from radioactive materials to generate a current. In the past
these batteries have been used in space missions as well as military applications.
New developments that hope to decrease their size will allow them to be used in
implanted medical devices and low voltage electrical devices.
Atomic batteries offer a lifespan relative to the half-life of the radioactive material being used, meaning that they can provide power for decades before they need to be replaced. The most common type of atomic battery uses betavoltaics. This means that a semiconductor material is used to absorb beta decay and generate a current.
Semiconductor materials are unfortunately decayed by beta particles. As a result the batteries donít last as long as they could. A team at the University of Missouri have designed an atomic battery that uses a liquid semiconductor, which allows it to degrade slower. Another design to solve this problem mixed the radioactive material with a phosphor (light-giving substance) and surround it with a transparent material. The semiconductor is replaced by a photoelectric surface of silicon. The radiation never passes beyond the transparent material and the light is collected and converted into electricity.
Cornell University has developed an atomic battery that uses a piezoelectric cantilever to produce electricity. Beta decay charges the cantilever which is then attracted to a positively charged thin film. When they touch, the two cantilevers receive equal charges and repel each other. The movement repeats and creates an electric charge.
Atomic batteries offer a lifespan relative to the half-life of the radioactive material being used, meaning that they can provide power for decades before they need to be replaced. The most common type of atomic battery uses betavoltaics. This means that a semiconductor material is used to absorb beta decay and generate a current.
Semiconductor materials are unfortunately decayed by beta particles. As a result the batteries donít last as long as they could. A team at the University of Missouri have designed an atomic battery that uses a liquid semiconductor, which allows it to degrade slower. Another design to solve this problem mixed the radioactive material with a phosphor (light-giving substance) and surround it with a transparent material. The semiconductor is replaced by a photoelectric surface of silicon. The radiation never passes beyond the transparent material and the light is collected and converted into electricity.
Cornell University has developed an atomic battery that uses a piezoelectric cantilever to produce electricity. Beta decay charges the cantilever which is then attracted to a positively charged thin film. When they touch, the two cantilevers receive equal charges and repel each other. The movement repeats and creates an electric charge.
Semiconductors
The following background information on semiconductors has been included because
of their direct use in atomic/nuclear batteries. Semiconductors are a ubiquitous part of modern society. They are the heart that
keeps the pulse of technology pumping as they are found in microprocessor chips
and transistors.
Anything that is computerized or uses radio waves depends on the utilization of
semiconductors.
Semiconductors are mostly composed of silicon or some sort of crystalline inorganic
solid (Group 4A has 4 valence electrons which offers an ease of uniformity when
crystallizing). This causes them to partially be considered resistors as they modify
the permeability of electricity between objects. To determine the magnitude of conductibility,
materials with different periodic properties are used. One particular property that
is of importance when considering material usage is the variance in electron mobility. Because of material consistency in a
semiconductor, the range of electricity that is allowed to pass through is made
controllable. This control allows for the alteration of operationsí speed,
temperature, and power of electronic devices.
In reference to atomic batteries, two main types of semiconductors are deliberated
upon: silicon and germanium, silicon being the more common of the two. Silicon is
easier to obtain and more popularly shipped and manufactured around the world. Silicon
in solar panels last, on average, up 20 years. If this is an accurate analogous for the durability
of silicon as semiconductors this would prove that it lasts longer and is the most
widely accepted choice. Silicon is also easier to process and experiences less leakage
than most materials commonly used in semiconductors. In terms of price, Silicon comes in as the cheapest material across
all grades. Regular grade silicon costs $0.50 per gram.
Germanium is an avid competitor in the conductive world. It is similar to silicon
in that they are periodic group neighbors. However, its availability is not as highly ranked as that of silicon
seeing as germanium is the fiftieth most commonly found element and silicon is the
second most abundant element in the Earthís crust.
#3 Cesium is not of vast economic importance.
http://www.democraticunderground.com/discuss/duboard.php?az=show_mesg&forum=115&topic_id=5609&mesg_id=20141
Applications of Caesium-137 in Soil Erosion and Sedimentation Studies
http://people.exeter.ac.uk/yszhang/caesium/welcome.htm
Betavoltaics
http://everything2.com/title/Betavoltaics
Commercial Purposes of Strontium-90
http://www.hps.org/publicinformation/ate/q219.html
Electron mobility in semiconductors
http://adsabs.harvard.edu/abs/1983PhRvB..28.4535N
Germanium
http://periodic.lanl.gov/32.shtml
How Semiconductors Work
http://www.howstuffworks.com/diode.htm
How Semiconductors Work
http://www.essortment.com/electronics-questions-semiconductors-work-29388.html
How long do solar panels last?
http://scitizen.com/future-energies/how-long-do-solar-panels-last-_a-14-2897.html
List of Radioactive Elements
http://www.buzzle.com/articles/list-of-radioactive-elements.html
Medical Uses
http://www.chem.duke.edu/~jds/cruise_chem/nuclear/medical.html
Neptunium
http://www.worldlingo.com/ma/enwiki/en/Neptunium
Nuclear battery keeps going, and going ...
http://www.msnbc.msn.com/id/7843868/ns/technology_and_science-science/
Physics of Uranium and Nuclear Energy
http://www.world-nuclear.org/education/phys.htm
Physics of Uranium and Nuclear Energy
http://www.world-nuclear.org/education/phys.htm
Plutonium-239
http://www.ausetute.com.au/nuclesum.html
Science: New Atomic Battery
http://www.time.com/time/magazine/article/0,9171,723820,00.html
Silicon
http://www.speclab.com/elements/silicon.htm
Smoke Detectors and Americium-241
http://www.chem.duke.edu/~jds/cruise_chem/nuclear/smoke.html
Technitium-99
http://www.ausetute.com.au/nuclesum.html
The Atomic Battery
http://www.technologyreview.com/Infotech/14548/?a=f
Tiny 'nuclear batteries' unveiled
http://news.bbc.co.uk/2/hi/technology/8297934.stm
Tiny atomic battery developed at Cornell could run for decades unattended, powering sensors or machines
http://www.news.cornell.edu/releases/Oct02/cantilever.ws.html
Uses of Cesium-137
http://www.ehow.com/list_7263688_uses-cesium_137.html
What is Betavoltaics?
http://www.wisegeek.com/what-is-betavoltaics.htm
