- The Vitruvian Plant: Powering the Future #10-2284
-
Background
History of Nuclear Energy Nuclear Agencies and Regulations Nuclear Power in the Public Eye Economics of Nuclear Energy Radioactivity Basics Nuclear Reactor Basics Current Reactor Designs Processing Radioactive Materials Remote Handling Uses of Radiation Archimedes Filter Technology New Generation Reactors
- Components
- Project
Radioactivity Basics
What is radioactivity?
When speaking about nuclear processes, the first terms that usually come to mind
are alpha, beta, and gamma decay. These are the three modes of natural radioactivity.
As a neutron in the nucleus decays into a proton, anti-electron neutrino, and an
electron, a beta particle is released. Since an electron is not a nuclear particle,
it must be expelled from the nucleus. Subsequently it behaves exactly like any "normal"
electron meaning in that it has the same mass and charge. During beta decay the
atom's atomic number increases by 1, while its number of nucleons remains unchanged.
An alpha decay occurs when an excited nucleus emits four nucleons - two protons
and two neutrons. This particle carries a positive charge of +2. Gamma decay occurs
when an excited nucleus "settles" to a lower energy level and releases its extra
energy in the form of a burst of electromagnetic radiation having energies ranging
from 41.4 keV to 414 keV. Gamma radiation always accompanies atomic fission when
an excited daughter nucleus, or decay product, is formed.
Nuclear fission refers to when an unstable radionuclide breaks down into two smaller daughter nuclei. This is usually done through the absorption of either a thermal (slow) neutron or a fast neutron. The terms fissile and fertile are used to describe this sensitivity. Fertile nuclei are fissionable when they are struck by a neutron. A fissile nucleus is one which, when it naturally fissions, produces one or more neutrons along with other radioactive nuclides that can subsequently emit alpha, beta, or gamma radiation.
There are three primary isotopes of uranium: 234U (0.005%), 235U (0.72%), and 238U (99.275%). 238U has a fertile nucleus which, when it absorbs neutrons having energies at 1 MeV or above1, transmutes into 239U which through subsequent beta decay produces 239Pu, an extremely fissile material.
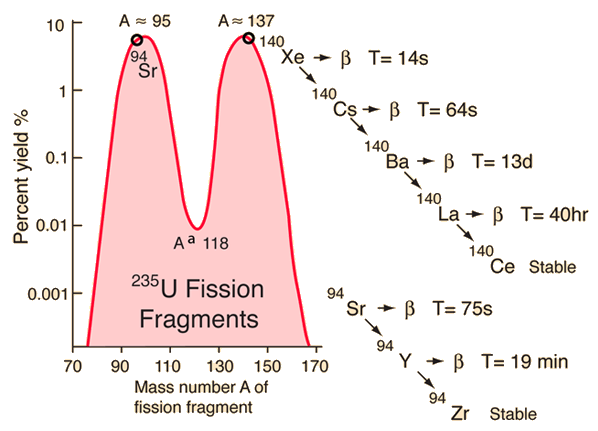
235U is a naturally occuring fissile nucleus which, when it absorbs a low energy, or thermal neutron, fissions about 85% of the time1. Otherwise, it becomes an excited 236U and fissions into a variety of daughter nuclei centering around the atomic masses of 95 and 137. A common pair of fragments are 140Xe and 94Sr with 2 fast neutrons. These daughter nuclei eventually decay into 94Zr and 140Ce. Nuclear power plants in the United States, France, Spain, use uranium dioxide (UO2) that is enriched at less than 5% in the 235U isotope1.
A common radioisotope found in spent nucelar fuel is Strontium-90. It has a half-life of 29.1 years and is used as a radioactive tracer since it chemical behavior is similar to that of calcium. Moreover, the heat generated by its decay has been converted into electricity for remote portable power supplies. Another common radioactive isotope is Cesium-137. It has a half-life of 30 years and is used in a myriad of industrial gauges. Its gamma rays are sometimes used for irradiation in cancer therapy as well as in some industrial applications.2
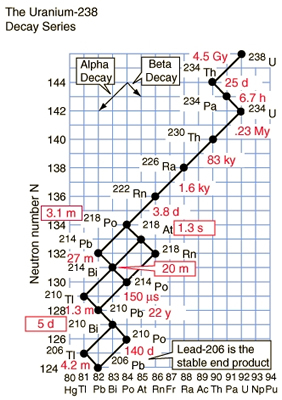
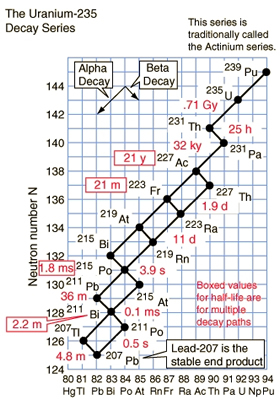
The term half-life corresponds to the statistical length of time it takes for half of a radioactive sample to decay. In the above diagram, various half-lives in seconds, minutes, days, and hours are denoted with a T. A half-life does not mean that the all of the radioistope is no longer present from the original sample.
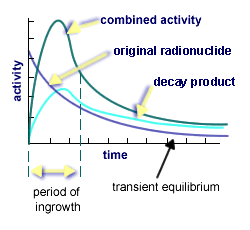
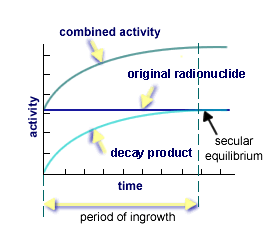
There are various categories of equilibrum in nuclear processes. As the original radioisotopes disintegrate, attention must be paid to the growth of decay products and the relative stability of the combined activity rates. There are two types of equilibrium states between the activity of original radionuclide and its decay products: transient, where both decay at approximately the same rate as shown on the left-hand graph; and, secular, where the half-life of the original is much greater than its decay products and the activity levels off as shown by the horizontal line on the right-hand graph.
Nuclear fission refers to when an unstable radionuclide breaks down into two smaller daughter nuclei. This is usually done through the absorption of either a thermal (slow) neutron or a fast neutron. The terms fissile and fertile are used to describe this sensitivity. Fertile nuclei are fissionable when they are struck by a neutron. A fissile nucleus is one which, when it naturally fissions, produces one or more neutrons along with other radioactive nuclides that can subsequently emit alpha, beta, or gamma radiation.
There are three primary isotopes of uranium: 234U (0.005%), 235U (0.72%), and 238U (99.275%). 238U has a fertile nucleus which, when it absorbs neutrons having energies at 1 MeV or above1, transmutes into 239U which through subsequent beta decay produces 239Pu, an extremely fissile material.
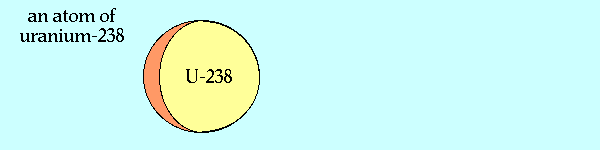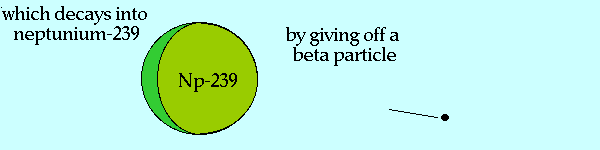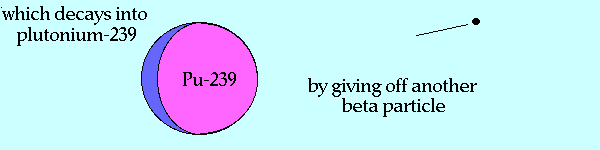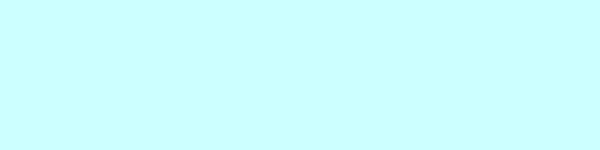
Animation courtesy of: The Government of India Department of Atomic Energy |
235U is a naturally occuring fissile nucleus which, when it absorbs a low energy, or thermal neutron, fissions about 85% of the time1. Otherwise, it becomes an excited 236U and fissions into a variety of daughter nuclei centering around the atomic masses of 95 and 137. A common pair of fragments are 140Xe and 94Sr with 2 fast neutrons. These daughter nuclei eventually decay into 94Zr and 140Ce. Nuclear power plants in the United States, France, Spain, use uranium dioxide (UO2) that is enriched at less than 5% in the 235U isotope1.
A common radioisotope found in spent nucelar fuel is Strontium-90. It has a half-life of 29.1 years and is used as a radioactive tracer since it chemical behavior is similar to that of calcium. Moreover, the heat generated by its decay has been converted into electricity for remote portable power supplies. Another common radioactive isotope is Cesium-137. It has a half-life of 30 years and is used in a myriad of industrial gauges. Its gamma rays are sometimes used for irradiation in cancer therapy as well as in some industrial applications.2
|
| Image courtesy of Hyperphysics at Georgia State University |
The term half-life corresponds to the statistical length of time it takes for half of a radioactive sample to decay. In the above diagram, various half-lives in seconds, minutes, days, and hours are denoted with a T. A half-life does not mean that the all of the radioistope is no longer present from the original sample.
There are various categories of equilibrum in nuclear processes. As the original radioisotopes disintegrate, attention must be paid to the growth of decay products and the relative stability of the combined activity rates. There are two types of equilibrium states between the activity of original radionuclide and its decay products: transient, where both decay at approximately the same rate as shown on the left-hand graph; and, secular, where the half-life of the original is much greater than its decay products and the activity levels off as shown by the horizontal line on the right-hand graph.
Natural and Enriched Uranium
The activity of uranium varies with its composition. In the case of natural uranium
ore, secular equilibrium exists for millions of years between both 238U and
and 235U and their decay products. In 2009, Australia, Kazakhstan,
and Canada accounted for 52% of the world’s uranium production.
When uranium ore is initially processed (reference the Shockwave video Responsible uranium extraction in Niger) into yellowcake,
or U3O8 (UO2 · 2UO3), its activity rate is drastically modified. Note on the following graph that the activity initially changes from ore's value of 175 Bq/gram, in the top uranium ore graph, to 25 kBq/gram on the bottom natural uranium graph. Eventually the activity rate doubles within 1 year to 50 kBq/gram, and finally reaches 175 kBq/gram within 1000 years. A becquerel(Bq) represents an activity rate of one disintegration/sec while a kBq represents a rate of 1000 disintegrations/sec.
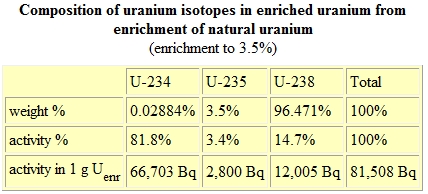
The two primary methods of enriching uranium hexafluoride use gaseous diffusion or a gas centrifuge. Both methods require expensive capital outlays for equipment, but the power required to operate the gas centrifuges is less expensive. Once the uranium-238 is enriched to 3-4% uranium-235, the activity rate for the enriched uranium fuel (UF6) originally processed from yellowcake is shown below.

|
|
|
Image courtesy
of World Information Service on Energy - Uranium Project
|
|
|
|
| Images courtesy of New World Encyclopedia | |
When uranium ore is initially processed (reference the Shockwave video Responsible uranium extraction in Niger) into yellowcake,
or U3O8 (UO2 · 2UO3), its activity rate is drastically modified. Note on the following graph that the activity initially changes from ore's value of 175 Bq/gram, in the top uranium ore graph, to 25 kBq/gram on the bottom natural uranium graph. Eventually the activity rate doubles within 1 year to 50 kBq/gram, and finally reaches 175 kBq/gram within 1000 years. A becquerel(Bq) represents an activity rate of one disintegration/sec while a kBq represents a rate of 1000 disintegrations/sec.
 A conveyor belt carrying processed yellowcake |

|
| Image courtesy of AREVA | Image courtesy of World Information Service on Energy - Uranium Project |
The two primary methods of enriching uranium hexafluoride use gaseous diffusion or a gas centrifuge. Both methods require expensive capital outlays for equipment, but the power required to operate the gas centrifuges is less expensive. Once the uranium-238 is enriched to 3-4% uranium-235, the activity rate for the enriched uranium fuel (UF6) originally processed from yellowcake is shown below.
|
| Image courtesy of World Information Service on Energy - Uranium Project |
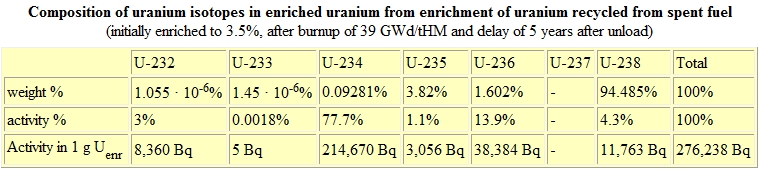
Uranium Fuel
The enriched UF6 is then
converted to UO2 powder and fabricated into uniform, ceramic
fuel pellets.
The pellets are next inserted into corrosive-resistance, Zircaloy-tubes and sealed by a "special welding process2 in an inert (helium) atmosphere as a cover gas and the helium is sealed into the tubes at about 400 psi pressure when manufacturing" is finished.2 The fuel rods are then grouped together to form fuel assemblies, or bundles.
Once placed inside a reactor, the fuel assemblies are rotated three times, at 18-month intervals. When their fuel is exhausted, the asemblies are removed from the reactor and moved to a cooling pond where they spend up to 5 years. During this time they can emit radiation at a rate of up to 5% of their original heat levels. After being removed from the cooling pond, they are sealed in steel or reinforced concrete dry casks for long term storage. Notice in the chart provided below that the activity rate for the spent nuclear fuel (SNF) has numerous additional padioactive pollutants from its time in the reactor.
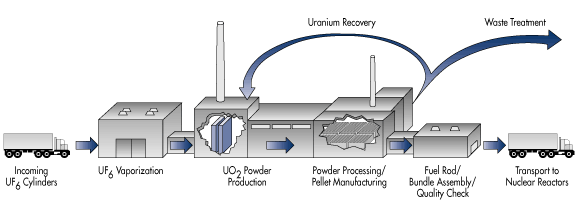
Image courtesy of the National Regulatory Commission |
The pellets are next inserted into corrosive-resistance, Zircaloy-tubes and sealed by a "special welding process2 in an inert (helium) atmosphere as a cover gas and the helium is sealed into the tubes at about 400 psi pressure when manufacturing" is finished.2 The fuel rods are then grouped together to form fuel assemblies, or bundles.
 U2O powder Image courtesy of Mitsubishi Nuclear Fuel Company, Limited  fuel pellets |
 fuel rod assemblies |
| Images courtesy of U.S. Energy Information Administration | |
Once placed inside a reactor, the fuel assemblies are rotated three times, at 18-month intervals. When their fuel is exhausted, the asemblies are removed from the reactor and moved to a cooling pond where they spend up to 5 years. During this time they can emit radiation at a rate of up to 5% of their original heat levels. After being removed from the cooling pond, they are sealed in steel or reinforced concrete dry casks for long term storage. Notice in the chart provided below that the activity rate for the spent nuclear fuel (SNF) has numerous additional padioactive pollutants from its time in the reactor.
|
| Image courtesy of World Information Service on Energy - Uranium Project |
Gamma-Matter Interaction
There are four possible results when a gamma photon passes through matter. The simplest
of these possibilities is that the photon passes through the object without hitting any particles.
A second options is that the photon will hit an atom and be completely absorbed in which case two reactions may occur depending on the binding energy of the electron and the energy of the gamma photon. If the the energy of the gamma photon is less than the binding energy, then the electron will move into an excited state but remain attached. If the energy of the gamma particle is more than the binding energy, then the photoelectric effect occurs and the electron is ejected. The kinetic energy of the electron can be determined by subtracting the binding energy of the electron from the energy of the gamma photon. The photoelectric effect is most likely to take effect when the difference between the binding energy and photon’s energy is small meaning the gamma photon must have low energy or the electron must have a high binding energy. Due to this, the photoelectric effect occurs most frequently when the energy of the gamma particle is less than half a megaelectron volt (MeV).
A third possibility is that the photon will hit an atom and be scattered. Depending on whether or not the photon deposits any energy, two things can happen. If the photon deposits no energy, which only occurs when the photon has little energy, then the gamma particle changes directions while losing very little energy. If the photon does deposit energy in an electron, however, the electron is ejected and the photon is scattered in a random direction with less energy than before which is known as Compton scattering. Compton scattering occurs most frequently when the gamma photon has between 0.5 MeV and 3.5 MeV. Depending on the energy of the emitted photon, it will go through either the photoelectric effect or Compton scattering until all of its energy is gone.
Finaly a process called pair production can occur. In pair production, the gamma particle interacts with the nucleus of an atom and converts energy into matter. Two particles are produced: an electron and a positron each with a mass equal to a rest mass energy of .51 MeV. This is why pair production cannot even occur unless the gamma photon has at least 1.02 MeV. It rarely occurs, however, until the energy levels reach several MeV. The excess energy is converted into kinetic energy that is divided evenly among the two particles. The two particles then zoom off through space either attracting electrons for the positron and repelling electrons for the electron until the positron is captured by an electron and the two particles are destroyed. When this happens two more photons with .51 MeV are produced and they undergo Compton scattering or the photoelectric effect until they lose all of their energy.
A second options is that the photon will hit an atom and be completely absorbed in which case two reactions may occur depending on the binding energy of the electron and the energy of the gamma photon. If the the energy of the gamma photon is less than the binding energy, then the electron will move into an excited state but remain attached. If the energy of the gamma particle is more than the binding energy, then the photoelectric effect occurs and the electron is ejected. The kinetic energy of the electron can be determined by subtracting the binding energy of the electron from the energy of the gamma photon. The photoelectric effect is most likely to take effect when the difference between the binding energy and photon’s energy is small meaning the gamma photon must have low energy or the electron must have a high binding energy. Due to this, the photoelectric effect occurs most frequently when the energy of the gamma particle is less than half a megaelectron volt (MeV).
A third possibility is that the photon will hit an atom and be scattered. Depending on whether or not the photon deposits any energy, two things can happen. If the photon deposits no energy, which only occurs when the photon has little energy, then the gamma particle changes directions while losing very little energy. If the photon does deposit energy in an electron, however, the electron is ejected and the photon is scattered in a random direction with less energy than before which is known as Compton scattering. Compton scattering occurs most frequently when the gamma photon has between 0.5 MeV and 3.5 MeV. Depending on the energy of the emitted photon, it will go through either the photoelectric effect or Compton scattering until all of its energy is gone.
Finaly a process called pair production can occur. In pair production, the gamma particle interacts with the nucleus of an atom and converts energy into matter. Two particles are produced: an electron and a positron each with a mass equal to a rest mass energy of .51 MeV. This is why pair production cannot even occur unless the gamma photon has at least 1.02 MeV. It rarely occurs, however, until the energy levels reach several MeV. The excess energy is converted into kinetic energy that is divided evenly among the two particles. The two particles then zoom off through space either attracting electrons for the positron and repelling electrons for the electron until the positron is captured by an electron and the two particles are destroyed. When this happens two more photons with .51 MeV are produced and they undergo Compton scattering or the photoelectric effect until they lose all of their energy.
1Professor William G. Vernetson, email dated 2/23/2011
2Professor William G. Vernetson, email dated 2/25/2011
Cesium
http://www.epa.gov/rpdweb00/radionuclides/cesium.html#wheredoes
Compton Scattering
http://www.ndt-ed.org/EducationResources/CommunityCollege/Radiography/Physics/comptonscattering.htm
E-4 UNF Dry Storage
https://app.fpl.com/energyEncounter/assets/learning-lab/unf/dry/index.jsp
E-4 UNF Wet Storage
https://app.fpl.com/energyEncounter/assets/learning-lab/unf/wet/index.jsp
Fission Fragment Example
http://hyperphysics.phy-astr.gsu.edu/hbase/nucene/fisfrag.html#c2
Fuel Assembly Manufacturing
http://www.mnf.co.jp/pages2/sei2.htm
Fuel Fabrication
http://www.nrc.gov/materials/fuel-cycle-fac/fuel-fab.html
Gamma Rays Interaction with Matter
http://www.scribd.com/doc/19387584/Gamma-Rays-Interactions-With-Matter
Geant4 to simulate Photoelectric, Compton, andPair production Events
http://www.physics.isu.edu/~tforest/Classes/NucSim/Day11/syed_hw12.pdf
Interaction of Electromagnetic Radiation and Matter
http://www.ndt-ed.org/EducationResources/CommunityCollege/RadiationSafety/theory/interaction.htm
Interaction of Radiation with Matter
http://www.sprawls.org/ppmi2/INTERACT/
Interaction of gamma radiation with matter
http://www.iasfbo.inaf.it/~amati/tesi/node31.html
Introduction to Nuclear Power
http://www.eia.doe.gov/cneaf/nuclear/page/intro.html
Nuclear Chemistry Worksheet #2: Energies of Nuclear Reactions
http://www.instruction.greenriver.edu/kmarr/Chem%20163/Ch163%20ALEs/1_C163%20POGIL%20ALEs/Chapter%2024%20Nuclear%20Chemistry%20Worksheets/29_Energies%20of%20Nuclear%20Reactions_Spr2010.pdf
Nuclear Chemistry: Uranium Production
http://www.chemcases.com/nuclear/nc-06.html
Pair Production
http://trshare.triumf.ca/~safety/EHS/rpt/rpt_2/node20.html
Photoelectric Effect
http://trshare.triumf.ca/~safety/EHS/rpt/rpt_2/node18.html
Processing: from ore to “yellow cake”
http://www.areva.com/EN/operations-677/uranium-processing-from-ore-to-yellow-cake.html?mediaDetail=814
Radioactive Decay
http://www.newworldencyclopedia.org/entry/Beta_ray
Radioactive Equalibrium
http://www.epa.gov/radiation/understand/equilibrium.html
Strontium
http://www.epa.gov/rpdweb00/radionuclides/strontium.html#discovered
Supply of Uranium
http://www.world-nuclear.org/info/inf75.html
Uranium Radiation Properties
http://www.wise-uranium.org/rup.html#UCONC