|
Mainland High School
Lord of the Trash Rings: ISTF 09-2004 |
||||
|
Home
Introduction Contest Components One Two  Product Product
Three Background Environmentalism History of Plastics Marine Laws Plastic Properties Pollution Laws Research Groups The Oceans Waste Management Project Assessment Team |
Waste Management Proposals
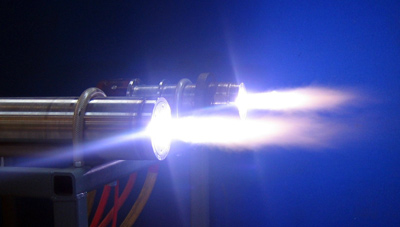
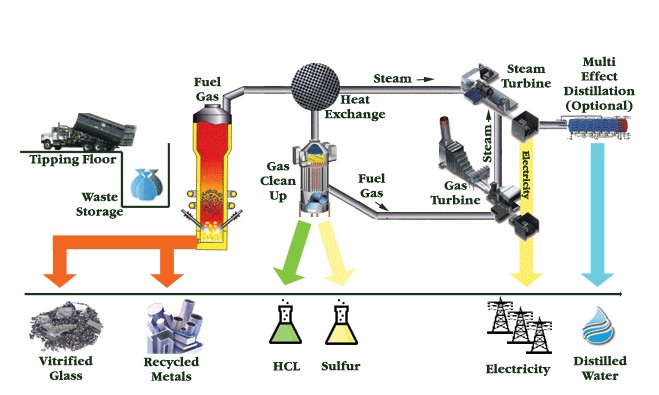
Plasma Gasification
Plasma arc gasification
is a processes of waste disposal in which waste is forced through an arc of ionized
particles of an inert gas, which heats the substance and the air around it to a
plasma. This breaks down all the molecular bonds in the substance down to the most
rudimentary level possible. The byproducts of this process are a hydrogen rich synthetic
gas that can be used for fuel and a very hard and durable substance called slag.
The exact make up of the gas and the slag is dependent on the substance being gasified.
The way this is accomplished is a small copper tube that's tapered at the end is filled with an inert gas. The copper is then electrified so that an arc of electricity jumps over the gap on the tapered end. This heats up the gas and causes it to expand, forcing it out of the hole. As the gas passes through the electric arc, it is superheated to a plasma, resulting in a "plasma torch". The waste is then pushed through this torch and obliterated. This whole whole process is undertaken inside of a sealed container so that the desirable gasses can be gathered and used elsewhere. The gasses produced are cooled and in the process their heat creates steam which turns turbines to recapture some of the energy input. The gas is then cleaned of dangerous components like chlorine or sulphur. What remains is a gas that can be burned and used for fuel. This "syngas" is composed of carbon monoxide, carbon dioxide and hydrogen gas. The slag produced is a glassy substance that traps any toxins like heavy metals, any organic toxins will have already been obliterated. This substance does not leach out these toxins, and has already has been tested and complies with EPA standards. If this slag is airated while cooling, it becomes rock wool. Rock wool is an excellent insulator and could replace other more harmful forms of insulation. This process has been used in many places to dispose of municipal solid waste, and has proven to be much more efficient than conventional incineration.
Pyrolysis
Pyrolysis is a catalyzed process of heating up a material so that the chemical bonds
will break. With plastic waste, the object is to break the polymers bonds at 400ēC.
The plastic then becomes a gas that is captured and cooled into a crude oil which
can then be further processed. Pyrolysis of plastic in the forms of PVC and polystyrene can release toxic gases. Polymer Energy boasts that the acceptable plastic does
not need to be sorted and that their process can handle a mixture of up to 25% other
waste products in the sample, including e-waste (computers). When using pyrolysis,
emissions are considerably
lower than those from incineration or gases released from land-fills.
In an e-mail dated Febuary 8th, 2010, Doug Woodring stated:
"One good thing is that is likely this plastic can be turned into fuel [pyrolysis],
maybe even on the boat itself while at sea, and this could help to subsidize the
larger cost of a clean up."
Dr. Kevin O'Connor at the University College Dublin, teamed up with Professor
Walter Kaminsky at the Uiversity of Hamburg, to develop a technique using pyrolysis
(to liquify the polystyrene into styrene oil) and bacterium to convert styrene oil
into a biodegradable plastic called PHA, polyhydroxalkanoate. This process would
allow polystyrene to no longer accumulate in landfills but become a fuel to produce
PHA, an environmentally-friendly plastic which when discarded does
degrade.
White-Rot Fungi
Enzymes
Enzymes are water-soluable, bio-molecules that are used in an organisms bodily
processes to speed up or catalyze biochemical reactions such as digestion and respiration.
They are made up of a protein and a cofactor which can be an organic group (prosthetic group),
a cation, positively charged metal ion (activator), or an organic molecule (coenzyme).
Enzmyes are commonly used in the food and brewery industry and as detergents. Three
methods of affecting enzyme-bonding techniques are: temperature, pH, and the concentration
of the enzyme.
Enzymes are that speed up reactions by lowering the activation energy required for the reaction to occur. Here is an analogy:
To fasten a screw into a piece of wood, many carpenters would agree it is much easier
to use a drill bit first on the wood to create a ideal hole/indent in order to easier
twist in the screw. Now if you can think of the screw as one reactant and the piece
of wood as the other then the drill bit would be the enzyme. Just like a reactant
with an enzyme, a hole in the wood requires a specifically shaped hole for the screw
to go in that it is neither too big nor too small but absolutely right. So in short
just like it is easier to secure a screw inside an already-drilled hole it is equally
easier for one reactant to react with another reactant when an enzyme can lower
the the required activation energy.
16-year old Daniel Burd won
top honors at Canada's Science Fair in Ottowa with his discovery of an enzyme
that can decompose plastic in as little as
3 months. The teen isolated two strains of bacteria,
Pseudomonas and Sphingomonas. He accomplished this by combining landfill dirt,
yeast and tap water, then added ground up plastic bags. The degradation process
was maximized at a temperature of 37ēC. He witnessed that the plastic indeed degraded
faster under these conditions than when it was simply discarded. In an interview,
Daniel stated that his process would be easy to replicate on an industrial scale,
all one needs is "a
fermenter, your growth medium, your microbes and your plastic bags." Daniel's
method is much
more environmentally friendly than other methods of biodegrading plastic with
the use of toxic heavy metal additives,
oxo-degradable plastics.
Synthetic Enzymes
Natural catalysts are used daily in the production of new medicines/other necessary
chemicals and food. Issues arise when trying to find a catalyst, because each molecule
has an individual shape. These individual shapes require the scientists to find
an individual catalyst each time, but creating a specified enzyme would prove to
bring much relief due to its unique abilities to self-adapt to its surrounding factors.
Chemists at
Ohio State University have now announced that they have the ability to produce
synthetic enzymes. This new technology will soon be applied to numerous applications
to shorten the time for
tedious trial-and-error experimenting that is required to find the ideal shape.
This remarkable breakthrough was made by a team of inventors including: Umit S. Ozkan, Lingzhi Zhang,
Xueqin Wang, and Sittichai Natasakhawat. The inventors have stated that they are
able to design an enzyme for any application necessary.
An enzyme that could facilitate the breakdown of plastic would help the complete disposal of plastics; but, it would need to be produced in considerable quantities. 16-year old discovers plastic-eating microbe: Is this the answer to our plastic problem? http://fakeplasticfish.com/2008/05/16-year-old-discovers-plastic-eating/ A Bittersweet Symphony called D2W http://fakeplasticfish.com/2008/01/bittersweet-symphony-called-d2w/ Aitkin County Plasma Gasification Study http://www.kauainetwork.org/_library/documents/kesp/technical%20libray/aitkincountyplasmagasificationassessment_final2.pdf A revolution in Recycling - Bugs that Eat Plastic http://www.ucd.ie/expertiseatucd/researchshowcase/2009/05MAY/plastic/index.html Dr Kevin O'Connor http://www.ucd.ie/research/success/featuredacademics/drkevinoconnor/ Eco-Friendly Way of Decomposing BPA-Containing Plastic http://www.sciencedaily.com/releases/2010/01/100127113753.htm Enzymes Function and structure http://www.rsc.org/education/teachers/learnnet/cfb/enzymes.htm Fungus Eats Enduring Plastic http://www.freerepublic.com/focus/news/1644760/posts Fungus is a Hot Solution to Disposing of BPA Laden Plastics http://www.treehugger.com/files/2010/02/fungus-is-a-hot-solution-to-disposing-of-bpa-laden-plastics.php#ch01 Florida county plans to vaporize landfill trash http://www.rexresearch.com/circeo/circeo.htm How Plasma Converters Work http://science.howstuffworks.com/plasma-converter3.htm InEnTec: Process Details http://www.inentec.com/pemtm-technology/process-details.html Ohio State University Synthetic Enzymes http://researchnews.osu.edu/archive/synzyme.htm Plasma Arc Gasification of Municipal Solid Waste http://www.energy.ca.gov/proceedings/2008-ALT-1/documents/2009-02-17_workshop/presentations/Louis_Circeo-Georgia_Tech_Research_Institute.pdf Plasma Gasification http://www.plasmagasification.com/ Plasma gasification: Clean Renewable Fuel Through Vaporization of Waste http://www.waste-management-world.com/index/display/article-display/368649/articles/waste-management-world/volume-10/issue-4/features/plasma-gasification-clean-renewable-fuel-through-vaporization-of-waste.html Polymer Energy FAQ's http://polymerenergy.com/faq Polymer Energy Technology http://polymerenergy.com/technology Pyrolysis as Recovering Value from Waste http://www.splainex.com/waste_recycling.htm Synthetic Enzymes a Reality http://www.scientistlive.com/European-Science-News/Biotechnology/Synthetic_enzymes_a_reality/20686/ Synthetic Molecules Emulate Enzyme Behavior For The First Time http://www.biology-online.org/articles/synthetic-molecules-emulate-enzyme-behavior.html Talking Trash with Dr. Louis J. Circeo http://blogs.gartner.com/dave_mccoy/2008/09/26/talking-trash-with-dr-louis-j-circeo/ Teen Decomposes Plastic Bag in Three Months http://www.wired.com/wiredscience/2008/05/teen-decomposes/ The Fungus among Us: Use of White Rot Fungi to Biodegrade Environmental Pollutants http://ehp.niehs.nih.gov/docs/1993/101-3/innovations.html The Future is Rotting Plastic http://scienceblogs.com/transcript/2006/06/the_future_is_rotting_plastic.php WCI student Isolates Microbe that Lunches on Plastic Bags http://news.therecord.com/article/354044 |