Background Information
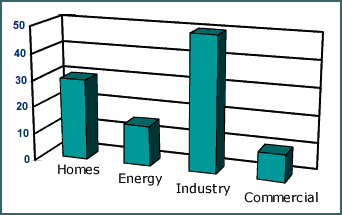
Methane is a naturally occurring fossil fuel. Most often, it is extracted from natural gas, which is made up of 90% methane, 5% propane and 5% mixed gases. Methane is also produced as a by-product of organic digestion. Natural gas is commonly used in homes around the United States as a way to heat and cool houses as well as to run many household appliances. It is especially popular because of its relative inexpense and its ability to be used while normal electrical lines are down. It is estimated that more than 50% of all the homes in the United States use natural gas (mostly methane). It is also estimated that more than 60% of all new homes built today have natural gas appliances installed. Home use of natural gas comprises 18% of the total market share and is described in detail below. The Major Categories of Methane and Natural Gas Usage The largest percentage of methane is used in the industrial world. However, almost all of that methane is used to produce heat or boil water. Neither of these represent an interest to our project. When the Environmental Protection Agency, or EPA, passed the clean air act in 1970, the power production market was forced to find cleaner, more environmentally friendly ways to produce power for the burgeoning population of the United States. One of the easier methods to achieve these new standards was to burn natural gas instead of coal or other highly pollutive substances. The methane rich natural gas is easily within the EPA's acceptable range for problematic gases. Many power plants are using natural gas as the burning fuel for their power plants. In the near future even more will be converting to natural gas. The Clean Air Act Amendments have caused many power plants to turn to natural gas in order to reduce their emissions of NOx and SO2, as it is the cleanest-burning of the widely used fuels. One of the largest foreseen problems is that the current rate of natural gas usage is causing reserves to plummet. The reserves are nowhere near exhausted, but they are predicted to be among the first of all fossil fuels to run out. The business section uses natural gas for a wide variety of applications. Businesses use natural gas for heat, power generation, appliance operation, and many other task specific applications. Unfortunately, these task specific applications vary greatly from business to business, so for our purpose, it can simply be said that the vast majority of methane gas in the commercial world is used for heating and power generation Methane (Natural Gas) can satisfy many needs in our everyday home energy necessity:
All of the information provided above was written with the help of Phoenix Natural Gas. All the images are used with their written permission. New and Upcoming Technologies A recent technology is currently under development that, theoretically, will use methane to power small household and office appliances. This breakthrough is a new branch of fuel cell technology and is given the same name. Fuel cells are electrochemical devices that convert the energy of the fuel directly to electrical energy. The three basic parts of fuel cells are the anode (negative electrode), cathode (positive terminal) and Proton Exchange Membrane (PEM). Inside a fuel cell, the PEM lies between the anode and the cathode. Hydrocarbon fuels (methane, ethane) are supplied to the anode , and oxygen, usually from air, is supplied to the cathode. Encouraged by a catalyst, the hydrogen atom from the fuel splits into a proton and an electron. Protons then pass through the PEM at the exact time that electrons take a separate path, thus forming an electrical current. (Fuel Cell Information Center) When the electrons reach the cathode, they recombine with the recently formed hydroxide ions into water vapor which is the only exhaust of the fuel cell. As in the combustion process, energy in the form of heat is a byproduct of combining fuel with oxygen. The fuel cell energy is released in the form of electric energy. Fuel cells are currently being used only in high-tech situations such as space exploration and military missions due to their high cost. However, recent inventions promise to make it possible to power your computer or your cellular phone with just a small bottle of methane and advanced fuel cell technology. (National Fuel Cell Research) Another new technology that promises to be a leading use for the future of methane and natural gas is the alternative vehicle market. Currently there are over 70,000 NGV's (natural gas vehicles) in America, over 1 million world-wide and 1,300 fueling stations for NGV's. However, of those 1,300 stations, only half are available for public use. Forty manufacturers are currently creating NGV's and 22% of all new bus orders are for those using natural gas engines. This is due in part to the cheaper costs of natural gas, which is about 1/3 the price of gasoline. It takes only 1.7 gallons of natural gas to equal the power of 1 gallon of gasoline. In addition, natural gas is also less polluting and has no evaporative emissions during fueling, which is where 50% of gasoline pollution comes from. Natural gas is also non-toxic and is not corrosive. It doesn't contaminate ground water supplies and, with its higher ignition temperature of 1200º F compared to the 600º F of gasoline, it is a safer fuel to use. Low emissions and economic feasibility promise to make NGV's a major source for alternative fuel vehicles. As gas prices rise alternative fuel vehicles will become more popular. It may not be long before these vehicles show up in your neighborhood. (Natural Gas Vehicle Coalition) Methane Safety Concerns Data sheets regarding properties of methane are supplied by OSHA (Occupational Safety and Health Administration) and the MSDS (Material Safety Data Sheet) on methane from National Welders. The most common method of controlling methane emissions is installing flares, mechanisms which burn the methane as it is being produced. Flares are recommended only when no other process is available. Flares save money by allowing institutions to avoid the cost of controlling hazardous air pollutant (HAP) and volatile organic compound (VOC) emissions, while reducing spending on safety risk prevention to operators (US Environmental Protection Agency). Instead of wastefully burning flares, methane can be utilized productively. Technology has progressed greatly since the 1970s when, during the energy crisis, methane usage was the trend and its explosive dangers were a major drawback. In comparison to the level of technology of yesteryear, the danger today is reduced by more mechanization of regulating devices and the increased ability for constant monitoring by computer control. The recent increase in job training, (FWPCOA) related to the handling of methane as well as other hazardous and volatile substances, also contributes to the safety of the process. When compared to the beneficial environmental impact of not using flares the, economic savings of using flares are negligible. Methane is a simple asphyxiate and extremely explosive. Leaks in the piping would be very detrimental to the safety of all concerned. Steel piping (Dr. Marcus Roediger) is often used in the handling of methane. The reason for this is because methane is inert to all metals and steel is quite durable, thus making it the industry standard for natural gas. Copper piping is relatively expensive, but a useable solution. However, PVC piping will also work. Due to the difficulty in spontaneously combusting methane under low pressures, well-sealed PVC piping works nicely for our purposes. Advanced Drainage System, Inc. represents a company which deals in making methane piping. Electrical Equipment Certification Service represents an explosion protection company in Britain. To the greatest degree possible, methane should be kept away from heat. Since the anaerobic tank must be heated to produce methane, this would seem to be a paradox. However, the desired temperature of the bacteria in the anaerobic tank is around room temperature, and the temperature needed for the combustion of methane is substantially higher than room temperature. |