Methyl tertiary-butyl ether, MTBE,
is a commonly used gasoline additive, possible carcinogen, and unburned, is a hazardous
air pollutant. It is a fuel oxygenate - a compound that contains oxygen within a chain of
carbon and hydrogen atoms. These are added to fuel to increase burning efficiency and
reduce emissions of harmful, toxic chemicals such as carbon monoxide and benzene, a known
carcinogen. MTBE is the most commonly used fuel oxygenate because it is the
most cost effective and has chemical properties that give it technical advantages over
other fuel oxygenates. (MTBE has a low water solubility, reactivity, and volatility in
comparison to other oxygenates. This makes it easier to add to gasoline.) The majority of
alternatives to MTBE don't increase efficiency or lower emissions as much, are in lower
supply, and are needed in larger amounts to produce the same effects.
MTBE is most harmful when it is used in the fuel for two-stroke engines. Two-stroke
engines are most commonly used in personal watercraft, primarily jet-skis. They release
25%-33% of their fuel unburned. This releases unburned MTBE into the water and because
MTBE volatizes, evaporates "out of" water, very quickly, MTBE is released into
the air as well. MTBE has carcinogenic effects when it is inhaled or orally consumed. In high doses in laboratory
situations, it has caused a significant increase in liver adenomas, benign liver tumors,
and carcinomas, malignant tumors, in female animal test subjects. After MTBE was
introduced to several cities in Alaska in 1992, complaints of
headaches, dizziness, throat irritation, nausea, and spaciness arose. The gasoline that
contained MTBE also smelled terrible. Alaska has gone back to using fuels that don't
contain MTBE since then. (Ironically, the levels of benzene, a known
carcinogen, in the blood levels of mechanics in Alaska has increased since MTBE was
removed from the fuel. Mechanics spent less time around the "funky" smelling
fuel when it contained MTBE and therefore, absorbed less benzene.)
The European Fuel Oxygenate Association (EFOA) disputes the health effects of MTBE. The
EFOA states that according to many toxicological studies, MTBE has been determined to not
be a carcinogen, mutagentic toxin, reproductive toxin, or neurotic toxin. Their site
quotes the WHO International Program on Chemical Safety (IPCS) as saying "based on
collective evidence, it appears unlikely that MTBE alone induces adverse acute health
effects in the general population under common exposure conditions." The "common exposure conditions" are
not defined. However, the National
Institute for Occupational Safety and Health attributes drowsiness, dizziness,
headache, weakness, and unconsciousness to the inhalation of MTBE and abdominal pain,
nausea, and vomiting to the ingestion of MTBE. This information is in correlation with the
symptoms experienced by the Alaskan residents when MTBE fuel was introduced.
The ways in which MTBE enters the water cycle are numerous. Underground storage tanks
of gasoline and other fuel leak into ground water. Fuel spills also release large amounts
of MTBE into the environment. The two-stroke engine releases large quantities of
noncombusted fuel, and large amounts of MTBE, into the environment as well. The majority
of the personal water craft on the market now are two-stroke engines. These popular
recreation vehicles emit large amounts of MTBE into our lakes, rivers, and reservoirs. The
amount of MTBE released into the environment by personal watercraft has
been determined to be one-tenth of a gallon every two hours. This amount of MTBE is enough
to contaminate thirteen million gallons of water.
Governor Gray Davis of California ordered that all MTBE had to be removed from gasoline
no later than December 31, 2002 in Executive
Order D-5-9. Governor George Pataki of New York passed legislation that bans the
"use, sales, and importation of MTBE" starting in 2004. The US Senate
Environment Committee approved an energy bill that would remove
MTBE from all gasoline by 2004.
Chemical Properties of MTBE
Chemical Formula: C5H12O
Chemical Structure: tertiary-butyl group + oxygen + methyl group
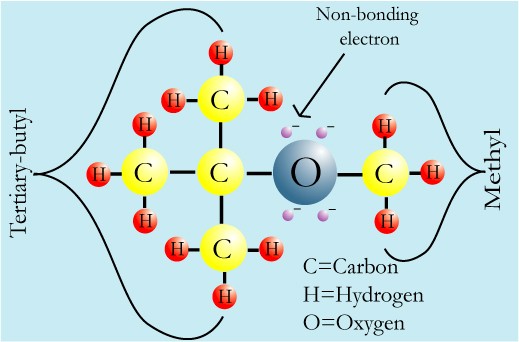
Each black bar symbolizes two shared, bonded electrons.
The non-bonding electrons on the oxygen make MTBE polar, more soluble
in water than the components in gasoline. Most other components in gasoline are purely
hydrocarbons. Hydrocarbons are non-polar. (This means that all the electrons in the
compound are being shared with the atoms. There are no non-bonding electrons. A polar
compound has non-bonding electrons. Polar compounds are only soluble in other polar
compounds, and the same goes for non-polar compounds.) Since water is polar, the other
gasoline components are not very soluble.
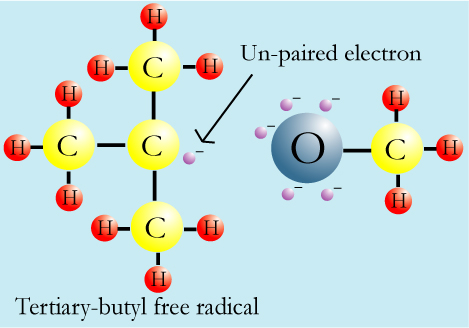
What makes MTBE a possible carcinogen is that the tertiary butyl [C(CH3)3]
as a free radical is a stable free radical. Free radicals contain atoms or groups of atoms
that have unpaired electrons. (Electrons are much more stable when they are in pairs.
Unpaired electrons "want " to "find" another electron and cause damage
to living things because they will strip that electron from other atoms.) This makes them
very reactive, and they can damage animal (including human) cells in such ways that
accelerate cancer, heart disease, and age-related diseases.